Today research activity is strongly driven by the non-invasive exploration of living bodies. In-Vivo testing is typically not an ideal option inside the human body. EM and Multiphysics simulation are the only way to analyze the complex field distribution inside the human body to ensure the functionality of the device as well as to understand and avoid hazards due to the power absorption inside the tissues.
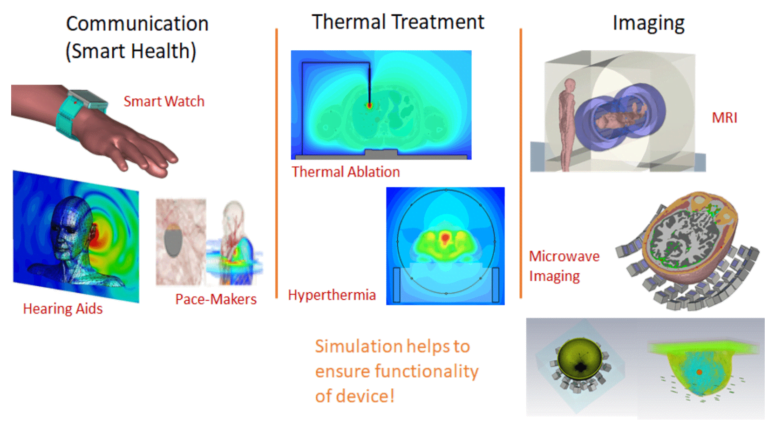
In this post, we analyze and answer all questions that arise while developing medical devices. The advent of 5G is upon us and the IoT market is continually evolving and anticipated to grow significantly to $24.46 billion by 2025.¹ The concept of IoT extends to Smart Health, where wearable and implantable devices, such as smartwatches, pacemakers, or hearing aids need to remain connected and functioning as intended in their environment all the time. The idea here is that different kinds of medical devices would be connected to each other and to the cloud where remote health monitoring and diagnosis would be seamlessly enabled. Another item that falls into this category, Abilify (a smart pill) is an arguably unusual choice for the first sensor-embedded medicine. The patch sends date and time of pill ingestion and the patient’s activity level via Bluetooth to a mobile phone application. The app allows patients to add their mood and the hours they have rested, then transmits the information to a database that physicians and others who have patients’ permission can access.
The Antenna is the critical enabling factor for all these connected medical devices. The antenna is embedded on a chip and that chip is placed inside or on the human body. The antenna needs to perform well in the body area network and effectively radiate in high dielectric environment of the human body. This technology helps to minimize the hospital time for people, especially the elderly. Senors could be worn on the body or implanted inside the body and are connected at all times to their smartwatch or mobile phone. In case of an emergency, the device would alert medical personnel and also provide useful real-time data to help in the diagnosis. This will revolutionize the life sciences industry in the coming years.
Concurrently, device manufacturers need to ensure the power absorbed by the human body adheres to compliance limits, which can be a challenging task. We also use electromagnetic energy to heat specific parts of tissue for cancer or tumor treatment so the cancerous cells that proliferate are terminated while the surrounding healthy cells remain unaffected. Because of the discomfort associated with invasively injecting electrodes inside the body, there is a lot of ongoing research to do this more non-invasively from the outside. This technique, known as Hyperthermia, consists of placing an array of antennas around the body in the region where the tumor has been identified. Typically a water bolus is included between the antenna array and the body in order to improve the transmission of energy from the antennas inside the tissues.
Electromagnetic waves are also used to image the internal parts of the body using traditional methods like Magnetic Resonance Imaging (MRI) and newer technologies like microwave imaging where an array of antennas are used in conjunction to transmit and receive signals.
Because these devices have chip and electronics components, medical devices electronic design analysis and EMC regulatory limits come into play. IEC 60601-1-2 and ANSI C63:19:2001 are different electromagnetic compatibility standards governed by the FDA for medical devices.² It is possible to investigate typical signal integrity issues like ringing, crosstalk, distortion, signal loss, and power supply loss and to analyze the complete signal channel from the transmitter to the receiver. The simulation results provide information about position and kind of distortion and how the eye diagram looks with and without equalization and clock recovery. Power integrity analysis deals with the power delivery between the power source and active devices. A clean and stable power delivery ensures good signal integrity and is a prerequisite to comply with the legal EMC limits.
So why perform simulation at all? The answer is pretty straightforward. First, to reduce time to market by avoiding costly and time-consuming prototype fabrication and testing. For example, in the case of an MRI system, the fields generated by the coils need to be homogeneous and penetrate the tissue sufficiently in order to image accurately and if the prototype does not meet this requirement, it would be a huge waste of money and effort. A major risk could arise with active implants like spinal modulators and pacemakers that have embedded electronics. We need to perform the analysis of how strong fields from MRI are interacting with the electronics to induce currents on the leads and PCBs – and could be potentially very harmful if the electromagnetic interference causes the malfunction of these devices. Currently, there are strict regulations that don’t allow patients with pacemakers and active implants to go anywhere near an MRI system – but in the future, this could be a hotspot of research where simulation will hold the key in analyzing if active implants could be certified as MR safe or not. To sum up, most medical devices that function in multiple physics environment not only involve electromagnetic, but also structural, thermal, and acoustic domains. 3DS solutions has analysis capabilities in all these domains.

Pankaj is an RF and Microwave engineer with more than 7 years of academic and industrial experience in modeling and simulation of different types of electromagnetic problems. He has worked in the areas of Antenna, Antenna Array, Filter Design, Microwave and RF circuits from GHz to THZ, bio-electromagnetics, EMI/EMC, EDA/Electronics and Low-Frequency Electromagnetic problems. He provides technical support and engineering services to customers and helps drive the growth of Vias3D electromagnetic solutions. He worked with Dassault Systems as a Solution Consultant and was responsible for Aerospace & Defense and High-Tech industry technical portfolios for the CST Studio Suite. He is a graduate of the Indian Institute of Technology, Delhi (IIT-D), where he studied Radio Frequency Design and Technology.
You can contact him at [email protected].
References:
- https://www.grandviewresearch.com/press-release/global-smart-medical-devices-market
- https://www.fda.gov/radiation-emitting-products/electromagnetic-compatibility-emc/emc-recognized-standards